
Chemistry, 03.12.2019 04:31 applereams
The chemical equation below shows the formation of aluminum oxide (al2o3) from aluminum (al) and oxygen (o2).
4al + 3o2 > 2al2o3
the molar mass of o2 is 32.0 g/mol. what mass, in grams, of o2 must react to form 3.80 mol of al2o3?
60.8
81.1
122
182

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2
You know the right answer?
The chemical equation below shows the formation of aluminum oxide (al2o3) from aluminum (al) and oxy...
Questions

Mathematics, 10.02.2021 01:40

Mathematics, 10.02.2021 01:40

Mathematics, 10.02.2021 01:40

Mathematics, 10.02.2021 01:40

Biology, 10.02.2021 01:40


Mathematics, 10.02.2021 01:40


History, 10.02.2021 01:40

Mathematics, 10.02.2021 01:40

Physics, 10.02.2021 01:40

Mathematics, 10.02.2021 01:40







Mathematics, 10.02.2021 01:40

Computers and Technology, 10.02.2021 01:40

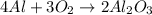
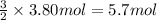 of oxygen gas.
of oxygen gas.

