
Chemistry, 03.12.2019 05:31 summerjoiner
Asample of 0.495 grams of solid khp is weighed into an erlenmeyer flask. this sample is titrated with a sodium hydroxide solution, and 28.56 ml of naoh are required to reach the endpoint. the sodium hydroxide solution is then used to titrate a sample of phosphoric acid of unknown concentration. it requires 29.88 ml of naoh to react with 10.33 ml of h3po4 solution. what is the concentration of the phosphoric acid?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Asample of 0.495 grams of solid khp is weighed into an erlenmeyer flask. this sample is titrated wit...
Questions




Mathematics, 15.12.2020 21:50

Mathematics, 15.12.2020 21:50




Mathematics, 15.12.2020 21:50

Mathematics, 15.12.2020 21:50


Mathematics, 15.12.2020 21:50

Biology, 15.12.2020 21:50

English, 15.12.2020 21:50


Mathematics, 15.12.2020 21:50

English, 15.12.2020 21:50


Mathematics, 15.12.2020 21:50

Mathematics, 15.12.2020 21:50

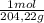 = 2,42x10⁻³ moles of KHP
= 2,42x10⁻³ moles of KHP

