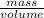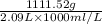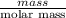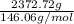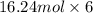Sulfur hexafluoride, a dense gas, is held in two separate containers in a storage room at an atmospheric pressure of 755 mmhg and 20.3 °c. the volume of container 1 is 2.09 l, and it contains 7.61 mol of the gas. the volume of container 2 is 4.46 l. determine the moles of f atoms in container 2 and the density of the gas at the conditions in the room

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3

Chemistry, 23.06.2019 15:40
The table below shows the freezing points of four substances. substance freezing point (°c) benzene 5.5 water 0 butane –138 nitrogen –210 the substances are placed in separate containers at room temperature, and each container is gradually cooled. which of these substances will solidify before the temperature reaches 0°c? benzene water butane nitrogen
Answers: 2
You know the right answer?
Sulfur hexafluoride, a dense gas, is held in two separate containers in a storage room at an atmosph...
Questions

Mathematics, 06.04.2021 01:00

Computers and Technology, 06.04.2021 01:00



Social Studies, 06.04.2021 01:00



Mathematics, 06.04.2021 01:00

Physics, 06.04.2021 01:00

Chemistry, 06.04.2021 01:00

Mathematics, 06.04.2021 01:00






Mathematics, 06.04.2021 01:00

Mathematics, 06.04.2021 01:00

Biology, 06.04.2021 01:00

Mathematics, 06.04.2021 01:00

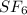 in container- 1 is as follows.
in container- 1 is as follows.