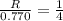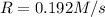Chemistry, 03.12.2019 05:31 caldonia2018
At a certain concentration of n2 and o3, the initial rate of reaction is 0.770 m / s. what would the initial rate of the reaction be if the concentration of n2 were halved? be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
You know the right answer?
At a certain concentration of n2 and o3, the initial rate of reaction is 0.770 m / s. what would the...
Questions

Chemistry, 02.08.2019 08:40


Mathematics, 02.08.2019 08:40

Chemistry, 02.08.2019 08:40


Physics, 02.08.2019 08:40

Mathematics, 02.08.2019 08:40

Health, 02.08.2019 08:40

Mathematics, 02.08.2019 08:40

Geography, 02.08.2019 08:40



Business, 02.08.2019 08:40

Mathematics, 02.08.2019 08:40

Mathematics, 02.08.2019 08:40



Health, 02.08.2019 08:40


![rate=k[N_2]^2[H_2]^2](/tpl/images/0400/5502/ac047.png)
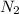 and
and 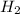 , the initial rate of reaction is 0.770 M/s. What would the initial rate of the reaction be if the concentration of
, the initial rate of reaction is 0.770 M/s. What would the initial rate of the reaction be if the concentration of ![0.770=k[N_2]^2[H_2]^2](/tpl/images/0400/5502/83364.png) ....(1)
....(1)![R=k(\frac{[N_2]}{2})^2[H_2]^2](/tpl/images/0400/5502/0c6c2.png) ....(2)
....(2)![\frac{R}{0.770}=\frac{k(\frac{[N_2]}{2})^2[H_2]^2}{k[N_2]^2[H_2]^2}](/tpl/images/0400/5502/4ab48.png)