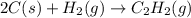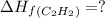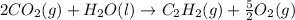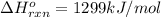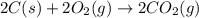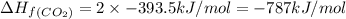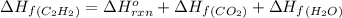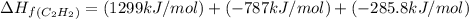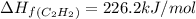The enthalpy of combustion of acetylene c2h2 is described by
c2h2 (g) + (5/2)o2 (g) >...

The enthalpy of combustion of acetylene c2h2 is described by
c2h2 (g) + (5/2)o2 (g) > > > > > > > co2 (g) + h2o (l) heat of reaction (rxn) = -1299kj/mol
calculate the enthalpy of formation of accetylene, given the following enthalpies of formation
standard formation [co2 (g)]= -393.5 kj/mol
standard formation [h2o (l)] = -285.8 kj/mol

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

You know the right answer?
Questions

Biology, 11.05.2021 16:50


Biology, 11.05.2021 16:50



Mathematics, 11.05.2021 16:50

Chemistry, 11.05.2021 16:50


Spanish, 11.05.2021 16:50



Mathematics, 11.05.2021 16:50




Spanish, 11.05.2021 16:50

Mathematics, 11.05.2021 16:50

Mathematics, 11.05.2021 16:50


 will be,
will be,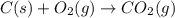
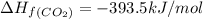
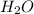 will be,
will be,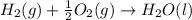
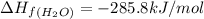
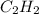 will be,
will be,