
Chemistry, 03.12.2019 06:31 Theacefamily123
The following reaction is exothermic.
2 s(s) + 3 o2(g) → 2 so3(g)
what can we say about the spontaneity of this reaction?
(a) spontaneous at all temperatures
(b) spontaneous only at high temperatures
(c) spontaneous only at low temperatures
(d) non spontaneous at all temperatures
(e) more information is need to predict if the reaction is spontaneous

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
The following reaction is exothermic.
2 s(s) + 3 o2(g) → 2 so3(g)
what can...
2 s(s) + 3 o2(g) → 2 so3(g)
what can...
Questions

Chemistry, 24.10.2020 08:00

History, 24.10.2020 08:10

Mathematics, 24.10.2020 08:10

Health, 24.10.2020 08:10

English, 24.10.2020 08:10

Mathematics, 24.10.2020 08:10

Mathematics, 24.10.2020 08:10

Mathematics, 24.10.2020 08:10


Mathematics, 24.10.2020 08:10

English, 24.10.2020 08:10

English, 24.10.2020 08:10


Biology, 24.10.2020 08:10

English, 24.10.2020 08:10

Health, 24.10.2020 08:10



World Languages, 24.10.2020 08:10

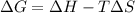
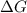 = Gibbs free energy
= Gibbs free energy 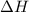 = enthalpy change
= enthalpy change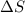 = entropy change
= entropy change 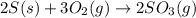

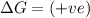 (at high temperature) (non-spontaneous)
(at high temperature) (non-spontaneous)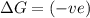 (at low temperature) (spontaneous)
(at low temperature) (spontaneous)

