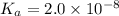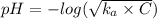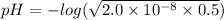Chemistry, 03.12.2019 17:31 Terrilady5
A0.50 m solution of an unknown acid has a ph = 4.0. of the following, which is the acid in the solution?
hocl (ka = 2.0 x 10-8)
hbr (strong acid)
hf (ka = 6.8 x 10-4)
c6h5oh (ka = 1.0 x 10-10)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
A0.50 m solution of an unknown acid has a ph = 4.0. of the following, which is the acid in the solut...
Questions


Mathematics, 08.10.2021 01:00


Mathematics, 08.10.2021 01:00

Biology, 08.10.2021 01:00

Mathematics, 08.10.2021 01:00

Geography, 08.10.2021 01:00

Mathematics, 08.10.2021 01:00

Mathematics, 08.10.2021 01:00


Law, 08.10.2021 01:00

Mathematics, 08.10.2021 01:00




History, 08.10.2021 01:00

Biology, 08.10.2021 01:00

Mathematics, 08.10.2021 01:00

Mathematics, 08.10.2021 01:00

Business, 08.10.2021 01:00

 ,
,