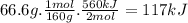Achemist measures the energy change ? h during the following reaction:
2fe2o3(s) ? 4fe...

Achemist measures the energy change ? h during the following reaction:
2fe2o3(s) ? 4feo(s) + o2(g) =? h560.kj
this reaction is.
(a) endothermic
(b) exothermic
suppose 66.6 g of fe2o3 react. will any heat be released or absorbed? |
(a) yes, absorbed
(b) yes released.
(c) no

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
Questions


Mathematics, 24.06.2020 02:01


Mathematics, 24.06.2020 02:01

Mathematics, 24.06.2020 02:01


Mathematics, 24.06.2020 02:01

Mathematics, 24.06.2020 02:01


Mathematics, 24.06.2020 02:01



Mathematics, 24.06.2020 02:01




Mathematics, 24.06.2020 02:01

Mathematics, 24.06.2020 02:01