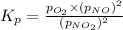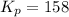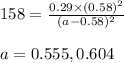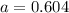Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1



Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
At 1000 k, a sample of pure no2 gas decomposes. 2 no2(g) equilibrium reaction arrow 2 no(g) + o2(g)...
Questions


History, 16.01.2020 04:31


Mathematics, 16.01.2020 04:31













Mathematics, 16.01.2020 04:31

Mathematics, 16.01.2020 04:31


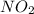 in the mixture is 0.58 atm and 0.024 atm respectively.
in the mixture is 0.58 atm and 0.024 atm respectively.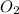 = 0.29 atm
= 0.29 atm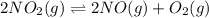
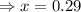
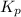 for above equation follows:
for above equation follows: