

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1


Chemistry, 23.06.2019 14:00
If you fill your car tire to a pressure of 32 psi (pounds per square inch) on a hot summer day when the temperature is 35°c (95°f), what is the pressure (in psi) on a cold winter day when the temperature is -15°c (5°f)? assume no gas leaks out between measurements and the volume of the tire does not change.
Answers: 1
You know the right answer?
Calculate the mass of oxygen (in mg) dissolved in a 5.00 l bucket of water exposed to a pressure of...
Questions

Advanced Placement (AP), 18.10.2020 03:01

History, 18.10.2020 03:01


Geography, 18.10.2020 03:01





Mathematics, 18.10.2020 03:01

Mathematics, 18.10.2020 03:01

Mathematics, 18.10.2020 03:01


English, 18.10.2020 03:01

Mathematics, 18.10.2020 03:01

Mathematics, 18.10.2020 03:01





Advanced Placement (AP), 18.10.2020 03:01

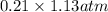
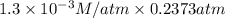
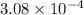 M
M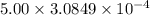
 mol
mol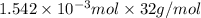 mol
mol 

