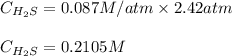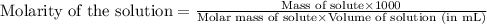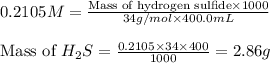Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 06:00
What does it mean for something to be dissolved in watera- it is submerged in water moleculesb-it is stirred in the water moleculesc- it is surrounded by water molecules d-it has water molecules added to it
Answers: 2

Chemistry, 23.06.2019 18:10
Which of the following changes would increase the molar concentration of the products in any chemical reaction atequilibrium?
Answers: 2
You know the right answer?
At 25.0 ⁰c the henry's law constant for hydrogen sulfide(h2s) gas in water is 0.087 m/atm. caculate...
Questions

Mathematics, 02.04.2020 02:19



Biology, 02.04.2020 02:19





Physics, 02.04.2020 02:19

Mathematics, 02.04.2020 02:19

Mathematics, 02.04.2020 02:19

Mathematics, 02.04.2020 02:19







Mathematics, 02.04.2020 02:20

Chemistry, 02.04.2020 02:20

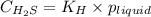
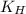 = Henry's constant =
= Henry's constant = 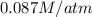
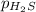 = partial pressure of hydrogen sulfide gas = 2.42 atm
= partial pressure of hydrogen sulfide gas = 2.42 atm