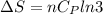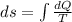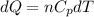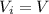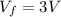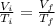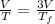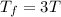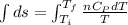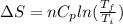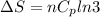Chemistry, 03.12.2019 21:31 squidmeat12
Asample consisting of n mol of an ideal gas undergoes a reversible isobaric expansion from volume vi to volume 3vi. find the change in entropy of the gas by calculating, ∫dq / t, where dq = ncpdt. (use the following as necessary: cp and n.)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1
You know the right answer?
Asample consisting of n mol of an ideal gas undergoes a reversible isobaric expansion from volume vi...
Questions



Chemistry, 18.01.2021 18:20



English, 18.01.2021 18:20


Physics, 18.01.2021 18:20

English, 18.01.2021 18:20


Social Studies, 18.01.2021 18:20


Geography, 18.01.2021 18:20




Mathematics, 18.01.2021 18:20



Mathematics, 18.01.2021 18:20