
Chemistry, 03.12.2019 22:31 allieballey0727
Given that h 2 ( g ) + f 2 ( g ) ⟶ 2 hf ( g ) δ h ∘ rxn = − 546.6 kj 2 h 2 ( g ) + o 2 ( g ) ⟶ 2 h 2 o ( l ) δ h ∘ rxn = − 571.6 kj calculate the value of δ h ∘ rxn for 2 f 2 ( g ) + 2 h 2 o ( l ) ⟶ 4 hf ( g ) + o 2 ( g )

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1
You know the right answer?
Given that h 2 ( g ) + f 2 ( g ) ⟶ 2 hf ( g ) δ h ∘ rxn = − 546.6 kj 2 h 2 ( g ) + o 2 ( g ) ⟶ 2 h 2...
Questions

Computers and Technology, 26.06.2019 05:00


History, 26.06.2019 05:00

Social Studies, 26.06.2019 05:00



Mathematics, 26.06.2019 05:00

History, 26.06.2019 05:00


History, 26.06.2019 05:00


History, 26.06.2019 05:00





Mathematics, 26.06.2019 05:00



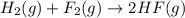
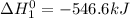 (1)
(1)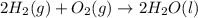
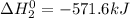 (2)
(2)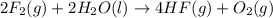
 (3)
(3)
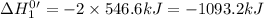 (1')
(1') .
.


