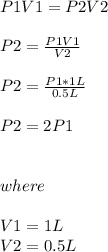Chemistry, 03.12.2019 23:31 lilybrok04
One liter of a gas is in a sealed chamber containing a moveable piston. if the piston is moved so that the volume of the gas is compressed to a volume of one-half liter, what will happen to the pressure on the gas? (assume the temperature is constant and no gas particles are lost.)
a)the pressure will be twice the original value.
b)the pressure will be half of the original value.
c)it would be impossible to move the piston since gases are not compressible.
d)the pressure will remain the same.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
One liter of a gas is in a sealed chamber containing a moveable piston. if the piston is moved so th...
Questions


Mathematics, 30.06.2019 03:30





Mathematics, 30.06.2019 03:30

Spanish, 30.06.2019 03:30


Biology, 30.06.2019 03:30


Biology, 30.06.2019 03:30


Mathematics, 30.06.2019 03:30

Mathematics, 30.06.2019 03:30



Mathematics, 30.06.2019 03:30


Mathematics, 30.06.2019 03:30