
Chemistry, 04.12.2019 00:31 anisagreen10
A0.158 g sample of magnesium metal reacts completely with 100.0 ml of 1.0 m hydrochloric acid in a coffee cup calorimeter. the temperature of the solution rose from 25.6°c to 32.8°c. what is ∆hrxn? assume the specific heat of the solution is 4.184 j/mol-k and the density is 1.0 g/ml.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
A0.158 g sample of magnesium metal reacts completely with 100.0 ml of 1.0 m hydrochloric acid in a c...
Questions

Mathematics, 06.11.2020 23:50

Mathematics, 06.11.2020 23:50

Mathematics, 06.11.2020 23:50



English, 06.11.2020 23:50

History, 06.11.2020 23:50



Mathematics, 06.11.2020 23:50

English, 06.11.2020 23:50

Geography, 06.11.2020 23:50

Computers and Technology, 06.11.2020 23:50

English, 06.11.2020 23:50

Mathematics, 06.11.2020 23:50



Arts, 06.11.2020 23:50

Chemistry, 06.11.2020 23:50

Biology, 06.11.2020 23:50

 =
= 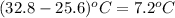
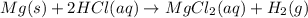
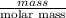
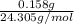
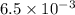
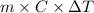
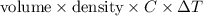
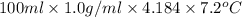
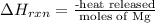
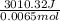
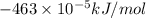 (as 1 kJ = 1000 J)
(as 1 kJ = 1000 J) kJ/mol.
kJ/mol. 

