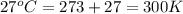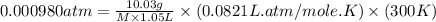Chemistry, 04.12.2019 00:31 zuleidysnegron
An aqueous solution of 10.03 g of catalase, an enzyme found in the liver, has a volume of 1.05 l at 27°c. the solution's osmotic pressure at 27°c is found to be 0.745 torr. calculate the molar mass of g/mol

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
An aqueous solution of 10.03 g of catalase, an enzyme found in the liver, has a volume of 1.05 l at...
Questions

Physics, 04.03.2021 20:30

Mathematics, 04.03.2021 20:30

Advanced Placement (AP), 04.03.2021 20:30

Mathematics, 04.03.2021 20:30


Computers and Technology, 04.03.2021 20:30

Mathematics, 04.03.2021 20:30





Chemistry, 04.03.2021 20:30

History, 04.03.2021 20:30




English, 04.03.2021 20:30


History, 04.03.2021 20:30

Mathematics, 04.03.2021 20:30

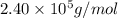
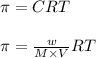
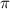 = osmotic pressure = 0.745 torr = 0.000980 atm (1 atm = 760 torr)
= osmotic pressure = 0.745 torr = 0.000980 atm (1 atm = 760 torr)