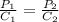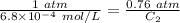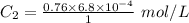Chemistry, 04.12.2019 00:31 fireman59937
The solubility of nitrogen gas at 25 ◦c and 1 atm is 6.8×10−4 mol/l. if the partial pressure of nitrogen gas in air is 0.76 atm, what is the concentration (molarity) of dissolved nitrogen?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
The solubility of nitrogen gas at 25 ◦c and 1 atm is 6.8×10−4 mol/l. if the partial pressure of nitr...
Questions






Mathematics, 10.11.2020 21:20






Mathematics, 10.11.2020 21:20

Computers and Technology, 10.11.2020 21:20

Chemistry, 10.11.2020 21:20

English, 10.11.2020 21:20


History, 10.11.2020 21:20


Health, 10.11.2020 21:20