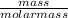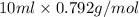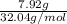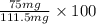Chemistry, 04.12.2019 00:31 Aydenj9613
Assume that you react 100 mg of benzoic acid with 10 ml of methanol and 10 microliters of sulfuric acid to produce methyl benzoate. write a balance chemical equation for this reaction. determine the limiting reagent and calculate a theoretical yield of both the ester and water. if you isolate 75 mg of methyl benzoate, what is the actual yield of the reaction?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
Assume that you react 100 mg of benzoic acid with 10 ml of methanol and 10 microliters of sulfuric a...
Questions

Mathematics, 09.11.2019 13:31


World Languages, 09.11.2019 13:31

Biology, 09.11.2019 13:31




Chemistry, 09.11.2019 13:31



Mathematics, 09.11.2019 13:31

Mathematics, 09.11.2019 13:31

Mathematics, 09.11.2019 13:31


Biology, 09.11.2019 13:31


Chemistry, 09.11.2019 13:31



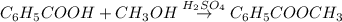
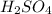 is very small so, that is catalytic amount of
is very small so, that is catalytic amount of