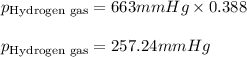Chemistry, 04.12.2019 01:31 andrejr0330jr
Amixture of nitrogen and hydrogen gases, at a total pressure of 663 mm hg, contains 3.46 grams of nitrogen and 0.156 grams of hydrogen. what is the partial pressure of each gas in the mixture? pn2 = mm hg ph2 = mm hg

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
Amixture of nitrogen and hydrogen gases, at a total pressure of 663 mm hg, contains 3.46 grams of ni...
Questions






Mathematics, 17.08.2021 02:20

Mathematics, 17.08.2021 02:20






Mathematics, 17.08.2021 02:20


Geography, 17.08.2021 02:20




Mathematics, 17.08.2021 02:20

Mathematics, 17.08.2021 02:20

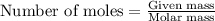
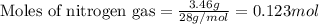
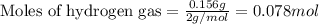
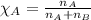 ......(1)
......(1)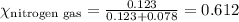
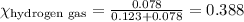
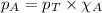 ......(2)
......(2)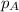 = partial pressure of substance A
= partial pressure of substance A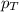 = total pressure = 663 mmHg
= total pressure = 663 mmHg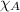 = mole fraction of substance A
= mole fraction of substance A