
Chemistry, 04.12.2019 02:31 dancer2814
Line spectra from all regions of the electromagnetic spectrum, including the paschen series of infrared lines for hydrogen, are used by astronomers to identify elements present in the atmospheres of stars. calculate the wavelength of the photon emitted when the hydrogen atom undergoes a transition from n = 5 to n = 3. (r = 2.179 x 10-18 j r = 1.096776 x 10^7 m-1) a. 205.1 nm b. 384.6 nm c. 683.8 nm d. 1282 nm e. > 1500 nm

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
Line spectra from all regions of the electromagnetic spectrum, including the paschen series of infra...
Questions


Mathematics, 14.01.2021 03:10

Arts, 14.01.2021 03:10



Chemistry, 14.01.2021 03:10

Mathematics, 14.01.2021 03:10


Mathematics, 14.01.2021 03:10




Mathematics, 14.01.2021 03:10

Geography, 14.01.2021 03:10


English, 14.01.2021 03:10


Mathematics, 14.01.2021 03:10


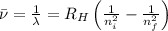
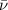 = Wave number
= Wave number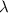 = Wavelength of radiation
= Wavelength of radiation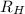 = Rydberg's Constant
= Rydberg's Constant = Higher energy level
= Higher energy level 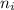 = Lower energy level
= Lower energy level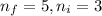
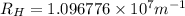
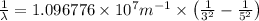
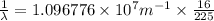
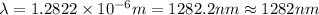
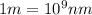 )
)

