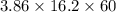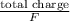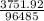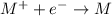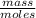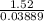Chemistry, 04.12.2019 02:31 tiamaharaj
Electrolysis of a molten salt with the formula mcl, using a current of 3.86 amp for 16.2 min, deposits 1.52 g of metal. identify the metal. (1 faraday = 96,485 coulombs) a) li b) na c) k d) rb e) ca

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
Electrolysis of a molten salt with the formula mcl, using a current of 3.86 amp for 16.2 min, deposi...
Questions
















History, 25.04.2020 04:06

Biology, 25.04.2020 04:06