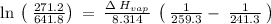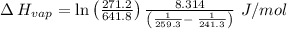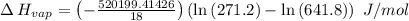Chemistry, 04.12.2019 03:31 gracynamos
The vapor pressure of the liquid so2 is measure at different temperatures. the following vapor pressure data are obtained. temp (k) pressure mmhg241.3 271.2259.3 641.8calculate the enthalpy of vaporization(delta h vap) in kj/mol for this liquid.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
The vapor pressure of the liquid so2 is measure at different temperatures. the following vapor press...
Questions

English, 04.09.2020 08:01



English, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01


Mathematics, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01

Biology, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01


Mathematics, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01


Advanced Placement (AP), 04.09.2020 08:01

History, 04.09.2020 08:01

Spanish, 04.09.2020 08:01

English, 04.09.2020 08:01

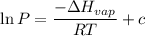
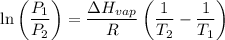
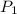 = 271.2 mmHg
= 271.2 mmHg
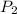 = 641.8 mmHg
= 641.8 mmHg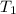 = 241.3 K
= 241.3 K 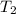 = 259.3 K
= 259.3 K