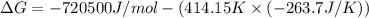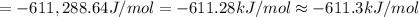Chemistry, 04.12.2019 03:31 ashcookie27
The value of delta g at 141.0 degrees celsius for the formation of phosphorous trichloride from its constituent elements,
p2(g) + 3cl2(g) > 2pcl3(g)
is kj/mol. at 25.0 degrees celsius for this reaction, delta h is -720.5 kj/mol, delta g is -642.9 kj/mol, and delta s is -263.7 j/k.
a.) -829.7
b.) 1.08 x 10^5
c.) 3.65 x 10^4
d.) -683.3
e.) -611.3

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
The value of delta g at 141.0 degrees celsius for the formation of phosphorous trichloride from its...
Questions

Mathematics, 03.06.2021 22:10







Mathematics, 03.06.2021 22:10


Mathematics, 03.06.2021 22:10


Arts, 03.06.2021 22:10

Biology, 03.06.2021 22:10

Biology, 03.06.2021 22:10

Mathematics, 03.06.2021 22:10

Mathematics, 03.06.2021 22:10



Mathematics, 03.06.2021 22:10