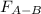Chemistry, 04.12.2019 03:31 christophercordero15
Two nonpolar organic liquids, hexane (c6h14) and heptane (c7h16), are mixed. do you expect δhsoln to be a large positive number, a large negative number, or close to zero? fill in the following sentence: δhsoln is determined by the relative magnitudes of the "old" solute-solute (δhsolute) and solvent-solvent (δhsolvent) interactions and the "new" solute-solvent (δhmix) interactions. in this process, the energy of mixing hexane and heptane (δhmix) is the energy of separating them individually (δhsolute + δhsolvent), so δhsoln is expected to be .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Two nonpolar organic liquids, hexane (c6h14) and heptane (c7h16), are mixed. do you expect δhsoln to...
Questions



History, 11.02.2021 07:50


Spanish, 11.02.2021 07:50



German, 11.02.2021 07:50

Mathematics, 11.02.2021 07:50

Mathematics, 11.02.2021 07:50




Mathematics, 11.02.2021 07:50

Mathematics, 11.02.2021 07:50


Mathematics, 11.02.2021 07:50

Mathematics, 11.02.2021 07:50


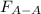 =
= 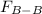 =
=