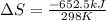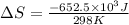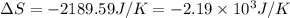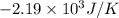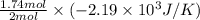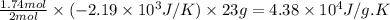Chemistry, 04.12.2019 07:31 pheonixhowls
Consider the reaction 2na(s) + 2h2o(l)2naoh(aq) + h2(g) using standard thermodynamic data at 298k, calculate the entropy change for the surroundings when 1.74 moles of na(s) react at standard conditions. s°surroundings = j/k g

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Consider the reaction 2na(s) + 2h2o(l)2naoh(aq) + h2(g) using standard thermodynamic data at 298k, c...
Questions

Computers and Technology, 15.12.2020 17:00

Mathematics, 15.12.2020 17:00

Physics, 15.12.2020 17:00





Mathematics, 15.12.2020 17:00



Computers and Technology, 15.12.2020 17:00



Mathematics, 15.12.2020 17:00




Geography, 15.12.2020 17:00


English, 15.12.2020 17:00

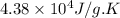
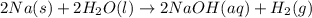
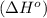 .
.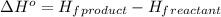
![\Delta H^o=[n_{NaOH}\times \Delta H_f^0_{(NaOH)}+n_{H_2}\times \Delta H_f^0_{(H_2)}]-[n_{Na}\times \Delta H_f^0_{(Na)+n_{H_2O}\times \Delta H_f^0_{(H_2O)}]](/tpl/images/0402/5848/95f1f.png)
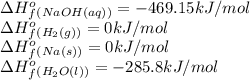
![\Delta H^o_{rxn}=[(2\times -469.15)+(1\times 0)]-[(2\times 0)+(2\times -285.8)]=-652.5kJ](/tpl/images/0402/5848/c5dc6.png)
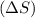 .
.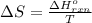
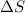 = change in entropy
= change in entropy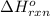 = change in enthalpy = -652.5 kJ
= change in enthalpy = -652.5 kJ