Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
60.0 ml of 0.322 m potassium iodide are combined with 20.0 ml of 0.530 m lead () nitrate. what is th...
Questions

Mathematics, 28.10.2020 22:00

English, 28.10.2020 22:00

History, 28.10.2020 22:00

English, 28.10.2020 22:00

Computers and Technology, 28.10.2020 22:00

Biology, 28.10.2020 22:00

Mathematics, 28.10.2020 22:00

History, 28.10.2020 22:00

Mathematics, 28.10.2020 22:00

English, 28.10.2020 22:00

Geography, 28.10.2020 22:00


Mathematics, 28.10.2020 22:00

Chemistry, 28.10.2020 22:00


Mathematics, 28.10.2020 22:00



History, 28.10.2020 22:00

Arts, 28.10.2020 22:00

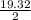 moles of Pb(NO₃)₂
moles of Pb(NO₃)₂