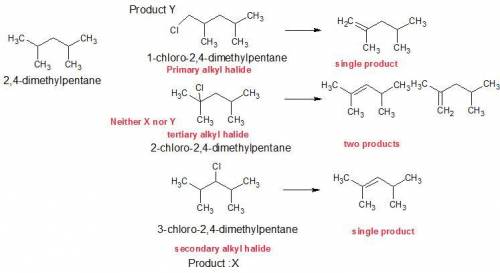
Compounds x and y are both c7h15cl products formed in the radical chlorination of 2,4-dimethylpentane. base-promoted e2 elimination of x and y gives, in each case, a single c7h14 alkene. both x and y undergo an sn2 reaction with sodium iodide in acetone solution to give c7h15i products; in this reaction y reacts faster than x. what is the structure of x?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
Compounds x and y are both c7h15cl products formed in the radical chlorination of 2,4-dimethylpentan...
Questions



History, 09.10.2019 16:50

History, 09.10.2019 16:50


Biology, 09.10.2019 16:50


Computers and Technology, 09.10.2019 16:50


Social Studies, 09.10.2019 16:50


Chemistry, 09.10.2019 16:50




Mathematics, 09.10.2019 16:50

English, 09.10.2019 16:50

Social Studies, 09.10.2019 16:50


Mathematics, 09.10.2019 16:50