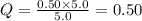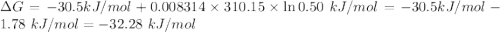Chemistry, 05.12.2019 03:31 chevysilverado3464
For which δg°rxn = –30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δgrxn in a biological cell in which [atp] = 5.0 mm, [adp] = 0.50 mm, and [hpo42–] = 5.0 mm.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2


Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
For which δg°rxn = –30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δgrxn in a biological...
Questions

Computers and Technology, 16.07.2019 15:00


Mathematics, 16.07.2019 15:00


Mathematics, 16.07.2019 15:00

Social Studies, 16.07.2019 15:00

Mathematics, 16.07.2019 15:00








Biology, 16.07.2019 15:00




Mathematics, 16.07.2019 15:00

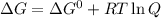
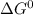 standard Gibbs energy
standard Gibbs energy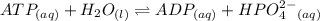
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0403/8875/ccdf0.png)
![[ATP]=5.0 mM](/tpl/images/0403/8875/1ddd2.png)
![[ADP]=0.50 mM](/tpl/images/0403/8875/91d08.png)
![[HPO_4^{2-}]=5.0 mM](/tpl/images/0403/8875/ff97d.png)