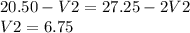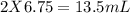Chemistry, 05.12.2019 06:31 Manuel2019
1. a mixture of naoh and na2co3 solution required 20.50 ml of 0.5 m hcl using
phenolphthalein as indicator. the same amount of alkali mixture when titrated using
methyl orange as an indicator required 27.25 ml of same acid. obtain an expression
for the volumes of acid required to neutralize (i) koh and (ii) na2co3 present in
solution

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2

Chemistry, 23.06.2019 10:30
Me soon im confused much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 1
You know the right answer?
1. a mixture of naoh and na2co3 solution required 20.50 ml of 0.5 m hcl using
phenolphthalein a...
phenolphthalein a...
Questions

English, 24.06.2019 13:00


Biology, 24.06.2019 13:00


English, 24.06.2019 13:00

Chemistry, 24.06.2019 13:00



Mathematics, 24.06.2019 13:00

Mathematics, 24.06.2019 13:00

Mathematics, 24.06.2019 13:00

Mathematics, 24.06.2019 13:00

Mathematics, 24.06.2019 13:00




Mathematics, 24.06.2019 13:00



 = 13.50 mL.
= 13.50 mL.

 (1)
(1)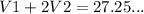 (2)
(2)