
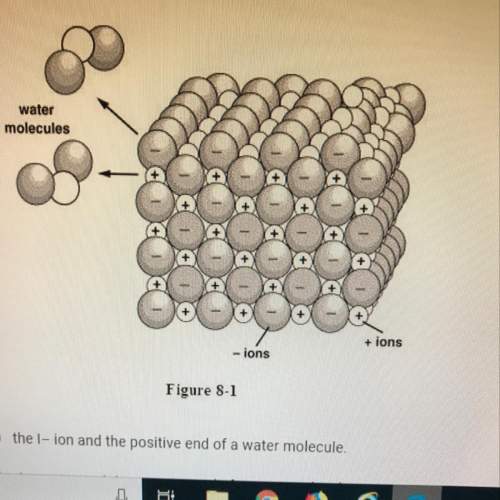
Iwill mark brainliest if the answer is study figure 8-1. when the ionic compound kl is dissolved in water, the k+
ions are pulled into solution by the attraction between
a. the i- ion and the positive end of a water molecule.
b. the k+ ion and the negative end of a water molecule.
c. the k+ and i- ions.
d. the i- ion and the negative end of a water molecule.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1
You know the right answer?
Iwill mark brainliest if the answer is study figure 8-1. when the ionic compound kl is dissolved in...
Questions

Mathematics, 24.08.2019 19:10

Mathematics, 24.08.2019 19:10



Mathematics, 24.08.2019 19:10

History, 24.08.2019 19:10


Mathematics, 24.08.2019 19:10


History, 24.08.2019 19:10

Biology, 24.08.2019 19:10


Mathematics, 24.08.2019 19:10

English, 24.08.2019 19:10



Mathematics, 24.08.2019 19:10

Mathematics, 24.08.2019 19:10

Mathematics, 24.08.2019 19:20

Mathematics, 24.08.2019 19:20



