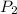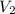Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
Afixed amount of gas at 25.0°c occupies a volume of 10.0 l when the pressure is 751 torr. use boyle'...
Questions

Mathematics, 31.08.2019 22:10

History, 31.08.2019 22:10

Computers and Technology, 31.08.2019 22:10



History, 31.08.2019 22:10

History, 31.08.2019 22:10

History, 31.08.2019 22:10

History, 31.08.2019 22:10

History, 31.08.2019 22:10

Social Studies, 31.08.2019 22:10

History, 31.08.2019 22:10

Chemistry, 31.08.2019 22:10



Mathematics, 31.08.2019 22:10

Mathematics, 31.08.2019 22:10

Mathematics, 31.08.2019 22:10

Biology, 31.08.2019 22:10

History, 31.08.2019 22:10

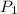
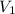 =
=