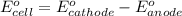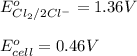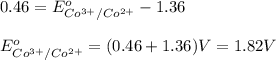Chemistry, 05.12.2019 18:31 eyeneedalife
The overall reaction 2co3+(aq) + 2cl–(aq) → 2co2+(aq) + cl2(g) has the standard cell voltage e°cell = 0.46 v. given e° = 1.36 v for the reaction cl2(g) + 2e– → 2cl–(aq), calculate the standard reduction potential for the following the half reaction at 25°c: co3+ + e– → co2+

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
The overall reaction 2co3+(aq) + 2cl–(aq) → 2co2+(aq) + cl2(g) has the standard cell voltage e°cell...
Questions

Mathematics, 11.11.2020 04:00


Social Studies, 11.11.2020 04:00


Mathematics, 11.11.2020 04:00

Mathematics, 11.11.2020 04:00



Mathematics, 11.11.2020 04:00

Mathematics, 11.11.2020 04:00

Mathematics, 11.11.2020 04:00

Mathematics, 11.11.2020 04:00


Physics, 11.11.2020 04:00




Mathematics, 11.11.2020 04:00


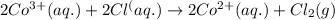
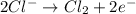
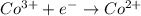
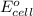 of the reaction, we use the equation:
of the reaction, we use the equation: