
Chemistry, 05.12.2019 18:31 madisonruh
Given the reactions, x ( s ) + 1 2 o 2 ( g ) ⟶ xo ( s ) δ h = − 505.9 kj xco 3 ( s ) ⟶ xo ( s ) + co 2 ( g ) δ h = + 199.3 kj what is δ h for this reaction? x ( s ) + 1 2 o 2 ( g ) + co 2 ( g ) ⟶ xco 3 ( s )

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1

Chemistry, 23.06.2019 14:00
Cassandra made a venn diagram to compare and contrast the two stages of cellular respiration. which belongs in the area marked x? energy is released. oxygen is used up. glucose is broken down. carbon dioxide is used up.
Answers: 1
You know the right answer?
Given the reactions, x ( s ) + 1 2 o 2 ( g ) ⟶ xo ( s ) δ h = − 505.9 kj xco 3 ( s ) ⟶ xo ( s ) + co...
Questions



English, 16.01.2021 01:00

English, 16.01.2021 01:00




Health, 16.01.2021 01:00

Advanced Placement (AP), 16.01.2021 01:00


Arts, 16.01.2021 01:00




Mathematics, 16.01.2021 01:00

Biology, 16.01.2021 01:00

History, 16.01.2021 01:00


Mathematics, 16.01.2021 01:00

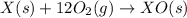
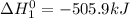 (1)
(1)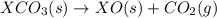
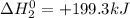 (2)
(2)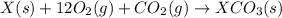
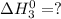 (3)
(3)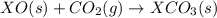
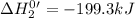 (2')
(2')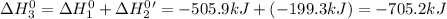
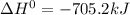 .
.

