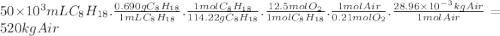Chemistry, 05.12.2019 19:31 mayamcmillan11
Consider stoichiometric combustion of gasoline and air. assume a full tank of gasoline holds 50 l (approximately 13.2 gallons), determine mass of air in kg required to combustion it. you can use isooctane c8h18 to approximate gasoline

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2
You know the right answer?
Consider stoichiometric combustion of gasoline and air. assume a full tank of gasoline holds 50 l (a...
Questions


Mathematics, 19.07.2021 14:10

Chemistry, 19.07.2021 14:10


Mathematics, 19.07.2021 14:10

Physics, 19.07.2021 14:20

Physics, 19.07.2021 14:20

Biology, 19.07.2021 14:20

Computers and Technology, 19.07.2021 14:20

Mathematics, 19.07.2021 14:20

History, 19.07.2021 14:20


English, 19.07.2021 14:20


Computers and Technology, 19.07.2021 14:20

Physics, 19.07.2021 14:20


Computers and Technology, 19.07.2021 14:20

Chemistry, 19.07.2021 14:20

Arts, 19.07.2021 14:20