
To identify a diatomic gas (x2), a researcher carried out the following experiment: she weighed an empty 5.8-l bulb, then filled it with the gas at 1.00 atm and 21.0 ∘c and weighed it again. the difference in mass was 6.7 g. identify the gas. express your answer as a chemical formula.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2


Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
To identify a diatomic gas (x2), a researcher carried out the following experiment: she weighed an...
Questions

Mathematics, 12.02.2021 18:00


Mathematics, 12.02.2021 18:00


Physics, 12.02.2021 18:00





Mathematics, 12.02.2021 18:00





Mathematics, 12.02.2021 18:00

Mathematics, 12.02.2021 18:00




 = (21 + 273) K = 294 K, mass = 6.7 g
= (21 + 273) K = 294 K, mass = 6.7 g
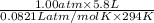



 .
.


