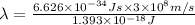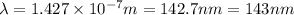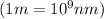Chemistry, 05.12.2019 20:31 arianawelsh123l
It takes 839./kjmol to break a carbon-carbon triple bond. calculate the maximum wavelength of light for which a carbon-carbon triple bond could be broken by absorbing a single photon.
round your answer to 3 significant digits in nm.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1


Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
It takes 839./kjmol to break a carbon-carbon triple bond. calculate the maximum wavelength of light...
Questions

Spanish, 18.12.2020 20:00

Mathematics, 18.12.2020 20:00

Mathematics, 18.12.2020 20:00


Biology, 18.12.2020 20:00

Geography, 18.12.2020 20:00


Mathematics, 18.12.2020 20:00

Mathematics, 18.12.2020 20:00

History, 18.12.2020 20:00

Chemistry, 18.12.2020 20:00


Chemistry, 18.12.2020 20:00


Mathematics, 18.12.2020 20:00

Mathematics, 18.12.2020 20:00

Chemistry, 18.12.2020 20:00

Mathematics, 18.12.2020 20:00


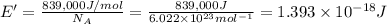
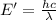 (Using planks equation)
(Using planks equation)