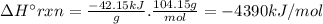Chemistry, 05.12.2019 20:31 leeamation31
Styrene, c8h8, is one of the substances used in the production of synthetic rubber. when styrene burns in oxygen to form carbon dioxide and liquid water under standard-state conditions at 25°c, 42.15 kj are released per gram of styrene. find the standard enthalpy of formation of styrene at 25°c.
(given: ? h°f[co2(g)] = –393.5 kj/mol, ? h°f[h2o(l)] = –285.8 kj/mol, ? h°f[h2o(g)] = –241.8 kj/mol)

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1


Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
Styrene, c8h8, is one of the substances used in the production of synthetic rubber. when styrene bur...
Questions



Spanish, 09.11.2019 02:31

Mathematics, 09.11.2019 02:31


Mathematics, 09.11.2019 02:31

Health, 09.11.2019 02:31

History, 09.11.2019 02:31


Social Studies, 09.11.2019 02:31

History, 09.11.2019 02:31


Biology, 09.11.2019 02:31

Mathematics, 09.11.2019 02:31

Mathematics, 09.11.2019 02:31



English, 09.11.2019 02:31

Mathematics, 09.11.2019 02:31