
Silver can be electroplated at the cathode of an electrolysis cell by the half-reaction. ag+(aq)+e−→ag(s) part a silver can be electroplated at the cathode of an electrolysis cell according to the following half-reaction: ag+(aq)+e−→ag(s) what mass of silver plates onto the cathode when a current of 8.5 a flows through the cell for 68 min ?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
Silver can be electroplated at the cathode of an electrolysis cell by the half-reaction. ag+(aq)+e−→...
Questions


Mathematics, 19.10.2021 01:00



Mathematics, 19.10.2021 01:00


Chemistry, 19.10.2021 01:00


Social Studies, 19.10.2021 01:00

SAT, 19.10.2021 01:00




History, 19.10.2021 01:00

Computers and Technology, 19.10.2021 01:00


Mathematics, 19.10.2021 01:00

Chemistry, 19.10.2021 01:00

Computers and Technology, 19.10.2021 01:00

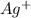 +e− ==> Ag(s)
+e− ==> Ag(s)

