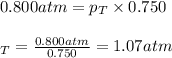Chemistry, 05.12.2019 21:31 happy121906
Acontainer has n and cl gases. if the mole fraction of n is 0.750 and it exerts 0.800 atm of pressure, what is the total pressure inside the container?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1



Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
Acontainer has n and cl gases. if the mole fraction of n is 0.750 and it exerts 0.800 atm of pressur...
Questions




Computers and Technology, 02.04.2020 03:54



Mathematics, 02.04.2020 03:54

Computers and Technology, 02.04.2020 03:54


Arts, 02.04.2020 03:54


Mathematics, 02.04.2020 03:54


History, 02.04.2020 03:54

Computers and Technology, 02.04.2020 03:54


History, 02.04.2020 03:54

Mathematics, 02.04.2020 03:54


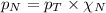
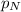 = partial pressure of nitrogen = 0.800 atm
= partial pressure of nitrogen = 0.800 atm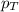 = total pressure
= total pressure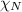 = mole fraction of nitrogen = 0.750
= mole fraction of nitrogen = 0.750