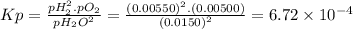Chemistry, 05.12.2019 22:31 jacxirylopez
The elementary reaction 2 h 2 o ( g ) − ⇀ ↽ − 2 h 2 ( g ) + o 2 ( g ) proceeds at a certain temperature until the partial pressures of h 2 o , h 2 , and o 2 reach 0.0150 bar , 0.00550 bar , and 0.00500 bar respectively. what is the value of the equilibrium constant at this temperature?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2


Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
The elementary reaction 2 h 2 o ( g ) − ⇀ ↽ − 2 h 2 ( g ) + o 2 ( g ) proceeds at a certain temperat...
Questions


History, 10.11.2021 20:00


Mathematics, 10.11.2021 20:00



Mathematics, 10.11.2021 20:00



English, 10.11.2021 20:00


Computers and Technology, 10.11.2021 20:00

History, 10.11.2021 20:00

Chemistry, 10.11.2021 20:00


Physics, 10.11.2021 20:00


Mathematics, 10.11.2021 20:00


English, 10.11.2021 20:00