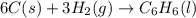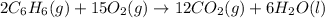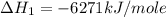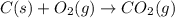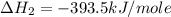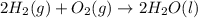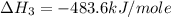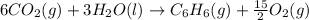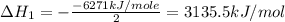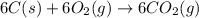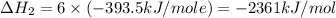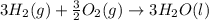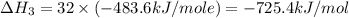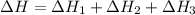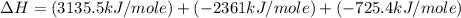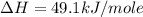Chemistry, 05.12.2019 23:31 carlinryan
Using the equations 2 c₆h₆ (l) + 15 o₂ (g) → 12 co₂ (g) + 6 h₂o (g)∆h° = -6271 kj/mol c (s) + o₂ (g) → co₂ (g) ∆h° = -393.5 kj/mol 2 h₂ (g) + o₂ (g) → 2 h₂o (g) ∆h° = -483.6 kj/mol determine the enthalpy for the reaction 6 c (s) + 3 h₂ (g) → c₆h₆ (l).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 23.06.2019 04:00
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1
You know the right answer?
Using the equations 2 c₆h₆ (l) + 15 o₂ (g) → 12 co₂ (g) + 6 h₂o (g)∆h° = -6271 kj/mol c (s) + o₂ (g)...
Questions


Mathematics, 06.10.2019 23:00

Mathematics, 06.10.2019 23:00


Geography, 06.10.2019 23:00

Mathematics, 06.10.2019 23:00


Spanish, 06.10.2019 23:00


Mathematics, 06.10.2019 23:00


Chemistry, 06.10.2019 23:00



Mathematics, 06.10.2019 23:00

English, 06.10.2019 23:00



History, 06.10.2019 23:00

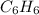 will be,
will be,