
Chemistry, 05.12.2019 23:31 voldermort9695
The mass defect for the formation of lithium-6 is 0.0343 g/mol. the binding energy for lithium-6 nuclei is kj/mol. enter your answer in exponential format (sample 1.23e-4) with two decimal places and no units.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1


Chemistry, 23.06.2019 11:00
Afraction can be converted to a decimal by dividing the denominator into the numerator. use a calculator. divide to convert the fractions from part a to decimals. then describe the pattern you see in the decimal.
Answers: 3
You know the right answer?
The mass defect for the formation of lithium-6 is 0.0343 g/mol. the binding energy for lithium-6 nuc...
Questions


Mathematics, 13.02.2022 20:20

Chemistry, 13.02.2022 20:20

Mathematics, 13.02.2022 20:20

Mathematics, 13.02.2022 20:30

Mathematics, 13.02.2022 20:30

English, 13.02.2022 20:30


Mathematics, 13.02.2022 20:30

Mathematics, 13.02.2022 20:30


Mathematics, 13.02.2022 20:30

Chemistry, 13.02.2022 20:30


Mathematics, 13.02.2022 20:30




Mathematics, 13.02.2022 20:30


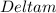 = Mass defect = 0.0343g/mol =
= Mass defect = 0.0343g/mol = 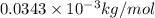 (Conversion factor:
(Conversion factor: 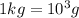 )
)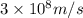
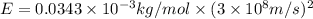
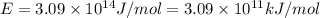 (Conversion factor:
(Conversion factor: 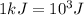 )
)

