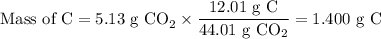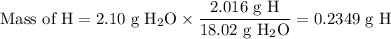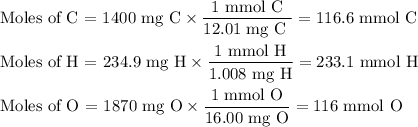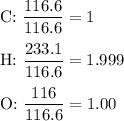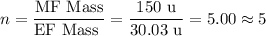Chemistry, 05.12.2019 23:31 aaleeyahprice
3.5g of a certain compound x, known to be made of carbon, hydrogen, and perhaps oxygen, and to have a molecular molar mass of 150g/mol is burned completely in excess oxygen, and the mass of the products carefully measured .
product
carbon dioxide 5.13g
water 2.10g
use this information to find the molecular formula of x

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1


You know the right answer?
3.5g of a certain compound x, known to be made of carbon, hydrogen, and perhaps oxygen, and to have...
Questions


History, 04.08.2019 07:50

Chemistry, 04.08.2019 07:50



Mathematics, 04.08.2019 07:50

Chemistry, 04.08.2019 07:50



Computers and Technology, 04.08.2019 07:50

Health, 04.08.2019 07:50