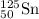Chemistry, 06.12.2019 00:31 ninilizovtskt
An atom of 125sn has a mass of 124.907785 amu. calculate the mass defect (deficit) in amu/atom. use the masses: mass of 1h atom = 1.007825 amu mass of a neutron = 1.008665 amu 1 amu = 931.5 mev give your answer to 4 significant figures and do not use e notation..

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

You know the right answer?
An atom of 125sn has a mass of 124.907785 amu. calculate the mass defect (deficit) in amu/atom. use...
Questions

Computers and Technology, 30.07.2019 04:20


Computers and Technology, 30.07.2019 04:20

Mathematics, 30.07.2019 04:20



Mathematics, 30.07.2019 04:20

Chemistry, 30.07.2019 04:20

Mathematics, 30.07.2019 04:20

Mathematics, 30.07.2019 04:20




Mathematics, 30.07.2019 04:20

English, 30.07.2019 04:20


History, 30.07.2019 04:20