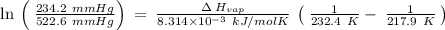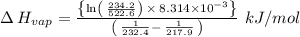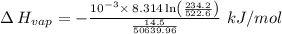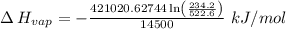Chemistry, 06.12.2019 00:31 graceaowen
The vapor pressure of the liquid nh3 is measured at different temperatures. the following vapor pressure data are obtained.
calculate the enthalpy of vaporization (? hvap) in kj/mol for this liquid.
p1 = 234.2 mmhg t1 = 217.9 k
p2 = 522.6 mmhg t2 = 232.4 k

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

You know the right answer?
The vapor pressure of the liquid nh3 is measured at different temperatures. the following vapor pres...
Questions

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Biology, 10.09.2020 17:01

Chemistry, 10.09.2020 17:01

English, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

World Languages, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

English, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

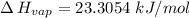
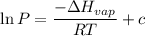
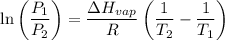
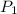 = 234.2 mmHg
= 234.2 mmHg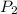 = 522.6 mmHg
= 522.6 mmHg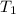 = 217.9 K
= 217.9 K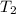 = 232.4 K
= 232.4 K