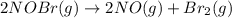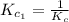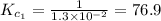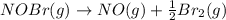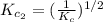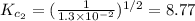Chemistry, 06.12.2019 02:31 taylorb9893
The equilibrium constant for the reaction 2no(g)+br2(g)⥫⥬==2nobr(g) is kc=1.3×10−2 at 1000 k. at this temperature does the equilibrium favor no and br2, or does it favor nobr? calculate kc for 2nobr(g)⥫⥬==2no(g)+br2(g). calculate kc for nobr(g)⥫⥬==no(g)+12br2(g).

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
You know the right answer?
The equilibrium constant for the reaction 2no(g)+br2(g)⥫⥬==2nobr(g) is kc=1.3×10−2 at 1000 k. at thi...
Questions


Biology, 28.08.2019 13:50





Mathematics, 28.08.2019 13:50


Mathematics, 28.08.2019 13:50

Mathematics, 28.08.2019 13:50


History, 28.08.2019 13:50

Social Studies, 28.08.2019 13:50

English, 28.08.2019 13:50


Spanish, 28.08.2019 13:50

English, 28.08.2019 13:50

Spanish, 28.08.2019 13:50

History, 28.08.2019 13:50

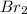 .
.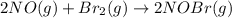
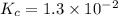 .
.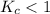 that means equilibrium lies to the left side. Thus, the equilibrium favors NO and
that means equilibrium lies to the left side. Thus, the equilibrium favors NO and