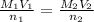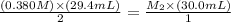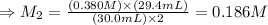Chemistry, 06.12.2019 02:31 NathanFrase6770
The amount of i − 3 ( aq ) in a solution can be determined by titration with a solution containing a known concentration of s 2 o 2 − 3 ( aq ) (thiosulfate ion). the determination is based on the net ionic equation 2 s 2 o 2 − 3 ( aq ) + i − 3 ( aq ) ⟶ s 4 o 2 − 6 ( aq ) + 3 i − ( aq ) given that it requires 29.4 ml of 0.380 m na 2 s 2 o 3 ( aq ) to titrate a 30.0 ml sample of i − 3 ( aq ) , calculate the molarity of i − 3 ( aq ) in the solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1
You know the right answer?
The amount of i − 3 ( aq ) in a solution can be determined by titration with a solution containing a...
Questions


Arts, 30.06.2019 10:30

Mathematics, 30.06.2019 10:30

Business, 30.06.2019 10:30



Social Studies, 30.06.2019 10:30

Social Studies, 30.06.2019 10:30



Mathematics, 30.06.2019 10:30

Physics, 30.06.2019 10:30



Mathematics, 30.06.2019 10:30

Geography, 30.06.2019 10:30

Geography, 30.06.2019 10:30


English, 30.06.2019 10:30

Biology, 30.06.2019 10:30