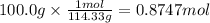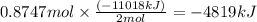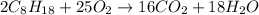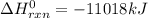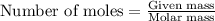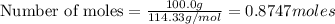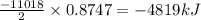Chemistry, 06.12.2019 02:31 autumnguidry7628
Using the following equation for the combustion of octane, calculate the heat associated with the combustion of 100.0 g of octane assuming complete combustion. the molar mass of octane is 114.33 g/mole. the molar mass of oxygen is 31.9988 g/mole.
2 c8h18 + 25 o2 → 16 co2 + 18 h2o δh°rxn = -11018 kj
a) -535.4 kj
b) -4819 kj
c) -602.3 kj
d) -385.5 kj
e) -11018 kj

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
Using the following equation for the combustion of octane, calculate the heat associated with the co...
Questions


Mathematics, 11.03.2020 21:28









Business, 11.03.2020 21:29