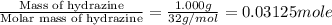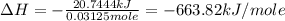Chemistry, 06.12.2019 03:31 isabeltorres5
A1.000 gram sample of the rocket fuel hydrazine (n2h4) is burned in a bomb calorimeter. the temperature rises from 24.62°c to 28.16°c. the heat capacity of the calorimeter (including the water) is 5860 j/°c. calculate the molar heat of combustion of hydrazine, in kj/mole.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
A1.000 gram sample of the rocket fuel hydrazine (n2h4) is burned in a bomb calorimeter. the temperat...
Questions

History, 25.06.2019 07:00


English, 25.06.2019 07:00

Mathematics, 25.06.2019 07:00



Physics, 25.06.2019 07:00

Chemistry, 25.06.2019 07:00

History, 25.06.2019 07:00

Mathematics, 25.06.2019 07:00


Computers and Technology, 25.06.2019 07:00


English, 25.06.2019 07:00






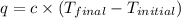
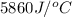
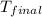 = final temperature =
= final temperature = 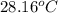
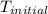 = initial temperature =
= initial temperature = 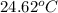

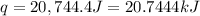
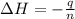
 = enthalpy change = ?
= enthalpy change = ?