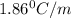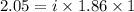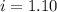Chemistry, 06.12.2019 03:31 Unkn0wn3815
You are given a 1.00 molal solution of an unknown solute dissolved in water and told that you must determine if the solute is a strong electrolyte, a weak electrolyte, or a nonelectrolyte. on testing, you find that the freezing point of the solution is -2.05 degrees celsius. is this enough information to answer the question? the molal freezing point depression constant for water is 1.86 °c/m. o no, this is not enough information o yes, the solute is a nonelectrolyte yes, the solute is a weak electrolyte yes, the solute is a strong electrolyte

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
You are given a 1.00 molal solution of an unknown solute dissolved in water and told that you must d...
Questions

Physics, 03.07.2019 09:00




Mathematics, 03.07.2019 09:00

Mathematics, 03.07.2019 09:00

Mathematics, 03.07.2019 09:00



Social Studies, 03.07.2019 09:00

History, 03.07.2019 09:00



Mathematics, 03.07.2019 09:00

History, 03.07.2019 09:00



Biology, 03.07.2019 09:00

Mathematics, 03.07.2019 09:00

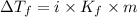
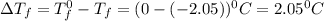 = Depression in freezing point
= Depression in freezing point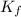 = freezing point constant =
= freezing point constant =